Human Genes, Microbes and Environment in CRC and IBD
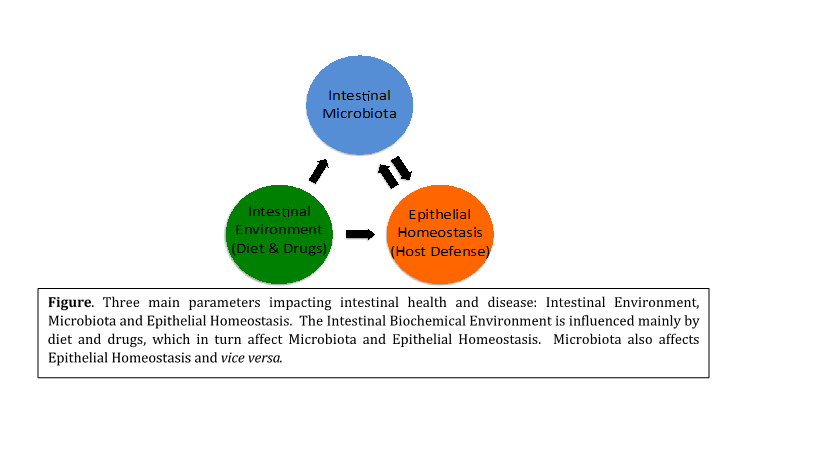
Host-microbe interactions control colonic dysbiosis, aberrant immune responses and intestinal epithelium damage, potentially predisposing for colorectal cancer (CRC). Most studies on CRC focus on tumor genetics, while more recently intestinal microbiota are studied in relation to health and disease. Nevertheless, neither tumor genetics nor microbiota composition alone may explain sporadic colon cancer development at an old age. To improve our understanding, we will need to pinpoint combinations of host, microbiota and lifestyle factors that correlate and predispose for the disease. Our work combines 3 basic components dictating intestinal health and disease (see Figure): (a) intestinal environment, primarily controlled by diet and orally administered chemicals; (b) intestinal microbiota, including potentially beneficial bacterial species (e.g. probiotics) and potentially harmful bacteria called pathobionts; and (c) epithelial homeostasis, that is preserved by host defense, including stem cell mediated regeneration and immunity. This combinatorial approach allows for a more complete and realistic analysis of intestinal balance and the parameters that are tilting it towards disease.
Mutations in K-Ras, APC, p53 and other genes are well-known CRC-contributing factors and accumulate in tumors over time. However, these mutations accumulate at different rates and do not necessarily exert the same effects in each individual. One could therefore reason that additional, non-genetic risk factors may act in concert with genetic changes to drive sporadic CRC as we age. Lifestyle is another factor contributing to CRC. The intestinal biochemical environment is shaped most prominently by dietary habits and by additional lifestyle factors, including cigarette smoking, heavy use of alcohol, infections, stress, obesity and physical inactivity. These factors may induce detrimental genetic or epigenetic alterations and changes in the microbiota. Interestingly, adopting healthy lifestyle habits at an old age, including following CRC diagnosis, improves survival prospects, indicating that prior detrimental alterations can be counteracted.
Similarly, various intestinal microbes have been suspected to contribute to CRC by impacting enterocyte proliferation and death, modifying host metabolism, or by disrupting immunological homeostasis. However, assigning a role for any of them as a causative agent of CRC is complicated. For example, establishing a causative relationship between Helicobacter pylori and gastric ulcers causing gastritis and cancer needed to satisfy most of Koch’s postulates, i.e. be found and isolated from ulcers, proven to cause disease when introduced to a healthy organism (Barry Marshall, the Nobel laureate himself), and tackled through antibiotic treatment for ulcer eradication. It is even more difficult to establish Koch’s postulates with a complex microbial community, especially if some microbes cannot be readily cultured.
Chronic inflammatory pathologies, such as inflammatory bowel disease (IBD), provide examples of how genetic and nongenetic factors intersect to orchestrate disease pathogenesis. Accumulating evidence highlights the impact of an exaggerated immune response to intestinal microbiota and dysbiosis, or aberrant microbial community composition, in the development of IBD and potentially cancer. The systemic inflammatory reactions to dysbiosis coupled with metabolic products of pathogenic bacteria establish a microenvironment rich in free radicals, DNA-damaging toxins, cytokines and growth factors that, collectively, foster tumor development. While IBD preexists in only a small number of people with CRC, the role of inflammation in cancer might be broader than previously thought. A subclinical form of inflammatory signaling that contributes to heightened epithelial regeneration, as pointed by studies in flies and mice, may instead contribute to many of the CRC cases.
The inheritance of IBD has been the focus of many epidemiologists because of the increased risk for IBD among relatives. 10 % of either UC or CD patients have been reported to have a family history of some type of IBD. Moreover, the concordance of CD and UC is much higher in genetically identical (monozygotic) versus non-identical (dizygotic) twins, which can be explained by genetic rather than environmental factors. Various genome wide association studies (GWAS) on UC and CD have identified genetic factors affecting IBD, namely, those related to the epithelial barrier function, such as mucins and matrix metalloproteinases, or oxidative stress related genes, such as ascorbate transporters, innate and adaptive immunity, regeneration and autophagy genes.
The human microbiome project revealed that bacterial species vary among individuals, but Bacteroides and Firmicutes and to a lesser extent Proteobacteria and Actinobacteria phyla prevail in the human intestine. Interestingly, Proteobacteria, including Enterobacteriaceae increase relative to Firmicutes and Bacteroides in IBD. In addition, dietary changes have been investigated in a number of studies on immigrant populations. Traditional diets of South Asian populations consist of mostly complex carbohydrates and minimal fats or sugars. However when foreign populations are exposed to western diets high in fat and sugar, in their lifetime or in subsequent generations, a significantly higher incidence of IBD is observed. A systematic review on food intake suggests a correlation between specific nutrients (fat, proteins, carbohydrates) and food groups (meats, vegetables, fruits) and the prevalence of IBD. For example, consumption of meat, saturated and total fat, and polyunsaturated fatty acids (PUFAS), such as low omega-3 to omega-6 ratio, correlate with increased risk for IBD. In contrast, the high intake of fiber, vegetables and fruits has the opposite effect.
