Diets relevant to CRC and IBD
Human colon provides a favorable environment for the successful colonization of anaerobic microbial where bacteria ferment non-digested dietary fibers or obtain nutrients from the secreted by the epithelium mucin, to produce energy. Particular members of gut microflora, including Clostridium clusters XIVa and IV (Faecalibacterium prausnitzii), Roseburia spp., Anaerostipes, Eubacterium and members of the genera Bifidobacterium and Lactobacillus, are considered beneficial due to the production of short chain fatty acids (SCFA) as final product of carbohydrate fermentation, the prevention of colonization of the gut by pathogens through releasing antimicrobial factors, the induction of epithelial-cells renewal, the secretion of essential for host lipopolysaccharides, vitamins and amino acids or the inhibition of the fermentation of the mucus glycosaminoglycans by pathogens that is involved in Inflammatory Bowel diseases progression (Leahy SC et al. 2005; Vlasova AN et al. 2016; Abigail Basson et al. 2016). SCFAs regulate several functions, such as cell mitosis and differentiation, secretion of hormone, metabolic homeostasis, and regulation of immune and inflammatory responses (Vinolo MAR et al. 2011; Wu J et al. 2012; Xiong Y et al. 2004; Tolhurst G et al. 2012; Kimura I et al. 2013; Miletta MC et al. 2014). Additionally, SCFAs indicate protective role against CRC and IBD via the modulation of colonic Tregs function and the inhibition of Nf-KB mediated expression of pro-inflammatory cytokines (Abigail Basson et al. 2016). SCFAs include: a) Butyric acid, which promotes colon motility, reduces inflammation, increases visceral irrigation, induces apoptosis, and inhibits tumor cell progression, b) Acetic acid, which indicates antibacterial properties, preventing pathogens (non acetic acid tolerance) colonization and biofilms formation and c) Propionic acid which is involved in gluconeogenesis and the control gut hormone release, food intake, lipogenesis, serum cholesterol levels, and carcinogenesis in some tissues (Hua V. Lin et al. 2012; Hosseini E 2011 et al. 2011). Consumption of prebiotics, such as some types of dietary fibers, resistant starch, fructo-oligosaccharides and beta-glucan assists the growth of beneficial bacteria (Probiotics) indicating protective role against IBD and CRC. Noteworthy, the presence of SCFA-producing bacteria is reduced in patients with IBD and CRC (Arianna K. et al. 2016).
Long chain monounsaturated fatty acids (LCMUFAs; MUFAs), such as olive oils (n-9) may influence chronic inflammations through the regulation of COX-2 expression, while long-chain polyunsaturated fatty acids (LCPUFAs; PUFAs), such as omega-3 (n-3), eicosapentanoic acid and docosahexanenoic acid, derived mostly from fish oil, indicate potent anti-inflammatory roles. On the contrary, omega-6 LCPUFAs are implicate in the initiation of acute inflammation. From medium-chain fatty acids (MCFA), lauric acid acts as TLR4 agonist triggering inflammation pathways but also creates monolaurin in gut, which is possible antimicrobial and antifungal. Increased high-fat SFA consumption enhances specific gut bacterial growth leading to overproduction of LPS and endotoxemia, which guide LTR4 triggering inflammation pathways that lead to acute inflammation (Abigail Basson et al. 2016).
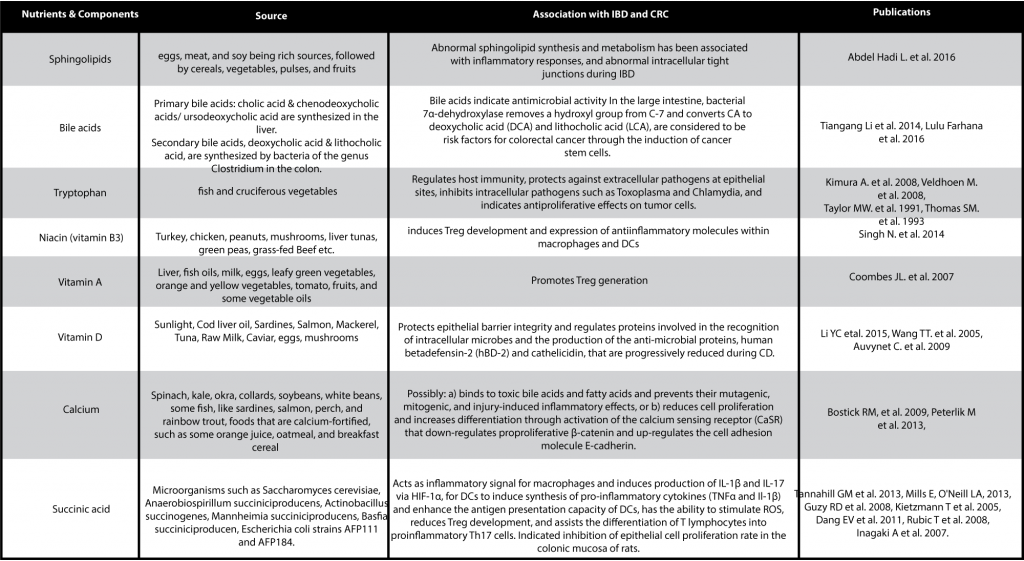
Other nutrients and compounds implicated in CRC and IBD are listed in the table below:
Additionally, Western diet, smoking, obesity, diabetes, alcohol consumption, and exposure to carcinogens have been considered essential risk factors for sporadic CRC development.
CRC treatment
- Surgery is the main treatment
- Radiation is barely used for the treatment of primary colon cancer
- Chemotherapy is used after surgery in the case of spread of tumor cells during surgery. Common regiments: 1. FOLFOX: 5-Fu, leucovorin and oxaliplatin. 2. Folfox: 5-Fu, leucovorin and irinotecan. 3.FOLFOXIRI: 5-Fu, oxaliplatin and irinotecan. 4. CapeOx: Capecitabin and oxaliplatin.
Publications:
- Abigail Basson, Ashley Trotter, Alex Rodriguez-Palacios, and Fabio Cominelli. Mucosal Interactions between Genetics, Diet, and Microbiome in Inflammatory Bowel Disease. Front Immunol. 2016; 7: 290.
- Arianna K. DeGruttola, B.S., Daren Low, Ph.D., Atsushi Mizoguchi, M.D., Ph.D., and Emiko Mizoguchi, M.D., Ph.D. Current understanding of dysbiosis in disease in human and animal models. Inflamm Bowel Dis. 2016 May; 22(5): 1137–1150.
- Anastasia N Vlasova, Sukumar Kandasamy, Kuldeep S Chattha, Gireesh Rajashekara, Linda J. Saif. Comparison of probiotic lactobacilli and bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Veterinary immunology and immunopathology2016
- Hua V. Lin, Andrea Frassetto, Edward J. Kowalik Jr, Andrea R. Nawrocki, Mofei M. Lu, Jennifer R. Kosinski, James A. Hubert, Daphne Szeto, Xiaorui Yao, Gail Forrest, and Donald J. Marsh. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut. PLoS One. 2012; 7(4): e35240.
- Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829.
- Leahy SC, Higgins DG, Fitzgerald GF, van Sinderen D. Getting better with bifidobacteria. J Appl Microbiol. 2005;98(6):1303-15.
-
Miletta MC, Petkovic V, Eblé A, Ammann RA, Flück CE, Mullis PE. Butyrate increases intracellular calcium levels and enhances growth hormone release from rat anterior pituitary cells via the G-protein-coupled receptors GPR41 and 43. PLoS One. 2014;9:e107388.
-
Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371.
-
Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876.
-
Wu J, Zhou Z, Hu Y, Dong S. Butyrate-induced GPR41 activation inhibits histone acetylation and cell growth. J Genet Genomics. 2012;39:375–384.
-
Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004;101:1045–1050
